Every year, millions of Americans fill prescriptions for generic drugs without ever knowing how they got approved. If you’ve ever picked up a pill bottle labeled generic instead of the brand name, you’ve benefited from one of the most successful public health programs in U.S. history. But how does the FDA make sure these cheaper pills work just as well? Let’s break it down-no jargon, no fluff, just what you need to know.
What Exactly Is a Generic Drug?
A generic drug is a copy of a brand-name medication. It has the same active ingredient, the same strength, the same way it’s taken (pill, injection, cream, etc.), and it works the same way in your body. The only differences? The name on the bottle and the price. Generic drugs cost, on average, 80-85% less than their brand-name counterparts. In 2023, about 90% of all prescriptions filled in the U.S. were for generics-but they made up only 23% of total drug spending.How Does the FDA Approve Them?
The FDA doesn’t start from scratch when approving a generic. Instead, manufacturers use a shortcut called the Abbreviated New Drug Application (ANDA). This process skips the expensive, years-long clinical trials that brand-name drug companies must run. Why? Because those trials were already done-years ago-when the original drug was first approved. The ANDA process focuses on two big things: pharmaceutical equivalence and bioequivalence.- Pharmaceutical equivalence means the generic has the same active ingredient, dose, and form as the brand-name drug. If the brand is a 10mg tablet taken by mouth, the generic must be exactly that.
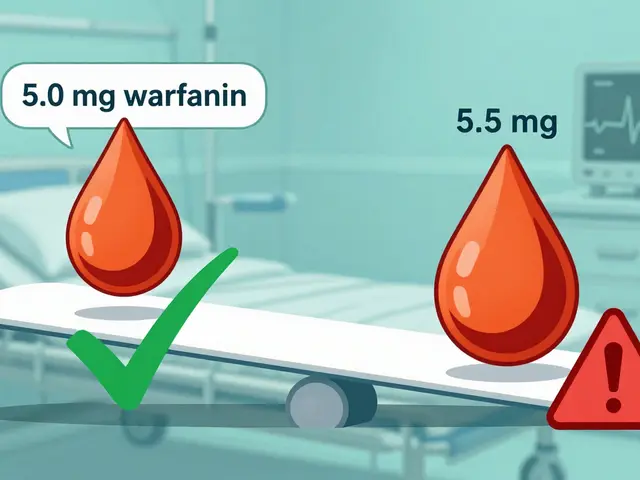
- Bioequivalence is the real test. It proves the generic gets into your bloodstream at the same rate and amount as the brand. This is tested in 24 to 36 healthy volunteers. Scientists measure how much of the drug enters the blood over time (called AUC) and how fast it peaks (called Cmax). The generic’s numbers must fall within 80% to 125% of the brand’s. That’s not a guess-it’s a strict scientific standard.
What Happens Behind the Scenes?
Before a generic drug hits the shelf, the manufacturer must prove their factory meets the same quality rules as the brand-name maker. The FDA inspects these facilities-about 1,500 inspections a year-and checks for compliance with Current Good Manufacturing Practices (cGMP). If a factory has issues, the FDA won’t approve the drug until they fix them. The application itself is massive. A simple tablet might involve 5,000 pages of data. A complex drug like an inhaler or eye drop can push that to 50,000 pages. The FDA has to review every bit of it. Thanks to the Generic Drug User Fee Amendments (GDUFA), most standard applications are reviewed in 10 months or less. Complex ones? They can take longer-sometimes over two years.Why Do Some Generics Take Longer to Come Out?
Not all generic drugs appear right after a brand-name patent expires. Sometimes, the brand company files extra patents to delay competition-a tactic called “evergreening.” These can push back generic entry by over three years, according to FTC data. Also, some drugs are just harder to copy. Inhalers, eye drops, and topical creams have complex formulations. The FDA calls these “complex generics,” and they’re the reason some patients wait longer. In fact, while only 15% of generic applications are for complex drugs, they cause nearly 40% of review delays.
Are Generics Safe? What About Side Effects?
Yes. The FDA requires generics to be as safe and effective as the brand. Over 87% of patients surveyed in a 2022 FDA study said they were satisfied with their generic medications. But there’s one small group that needs extra care: people taking narrow therapeutic index (NTI) drugs. These are medications where even tiny changes in blood levels can cause problems-like warfarin (a blood thinner) or levothyroxine (for thyroid issues). Some patients report feeling different when switching between generic brands. That’s not because the generics are unsafe-it’s because their bodies are sensitive. If you’re on one of these drugs, talk to your doctor before switching. Many pharmacists will keep you on the same generic brand unless you say otherwise.Who Makes These Drugs?
About 150 companies make generic drugs in the U.S. The biggest players are Teva, Viatris, and Amneal. But many generics are made overseas-mostly in India and China. The FDA inspects those factories too. In fact, more than half of the facilities that make generic drugs are outside the U.S. The FDA doesn’t treat them differently. If they don’t meet the standards, the drug doesn’t get approved.How Is This Different from Biosimilars?
You might hear about “biosimilars” and wonder if they’re the same as generics. They’re not. Biosimilars are copies of biological drugs-like insulin, cancer treatments, or rheumatoid arthritis meds. These are made from living cells, not chemicals, so they can’t be exact copies. The approval process for biosimilars is more like the brand-name process, and there are only about 41 approved as of late 2023. In contrast, the FDA has approved over 15,000 generic drugs.
What Should You Do as a Patient?
- Ask your doctor or pharmacist if a generic is available for your prescription.
- Don’t assume a generic is “weaker.” It’s not. It’s the same medicine, just cheaper.
- If you’re on a critical medication like warfarin or levothyroxine, stick with the same generic brand unless your doctor advises otherwise.
- If you notice a change in how you feel after switching generics, tell your provider. It’s rare, but it can happen.
- Check the FDA’s Orange Book online to see which generics are approved for your brand-name drug.
What’s Next for Generic Drugs?
The FDA is working on new tools to speed up approval of complex generics. In 2023, they approved the first generic version of EpiPen-a huge win after 15 years of trying. By 2025, they plan to use artificial intelligence to help review applications faster. Meanwhile, over $250 billion in brand-name drugs are set to lose patent protection by 2027, which means more generics are coming. The system isn’t perfect. It’s slow for complex drugs. It’s sometimes held up by legal battles. But overall, it works. It saves patients billions every year. And it lets people who couldn’t afford their meds now get them.Are generic drugs as effective as brand-name drugs?
Yes. The FDA requires generic drugs to have the same active ingredient, strength, dosage form, and route of administration as the brand-name drug. They must also prove bioequivalence-meaning they deliver the same amount of medicine into your bloodstream at the same rate. Thousands of studies and decades of real-world use confirm they work the same way.
Why are generic drugs so much cheaper?
Brand-name drug companies spend an average of $2.6 billion to develop a new drug, including years of clinical trials. Generic manufacturers don’t repeat those trials-they rely on the brand’s data. Their costs are around $5-10 million per drug. That savings gets passed on to patients and insurers.
Can I trust generics made outside the U.S.?
Yes. The FDA inspects all manufacturing facilities-whether they’re in the U.S., India, China, or elsewhere. They use the same standards. In fact, more than half of generic drugs sold in the U.S. are made overseas. If a facility fails inspection, the FDA blocks the drug from entering the market.
Why do some people say they feel different on a generic?
For most people, there’s no difference. But for those taking narrow therapeutic index drugs-like warfarin, levothyroxine, or certain seizure meds-tiny changes in blood levels can matter. Switching between different generic brands might cause a slight variation in how you feel. If this happens, tell your doctor. You can often ask your pharmacist to keep you on the same generic brand.
How long does it take for a generic to come out after a brand-name drug’s patent expires?
For simple drugs like pills or capsules, generics usually arrive about 14 months after patent expiration. But for complex drugs-like inhalers or eye drops-it can take much longer, sometimes several years, because proving bioequivalence is harder. Legal challenges from brand companies can also delay entry.
Does the FDA monitor generic drugs after they’re on the market?
Yes. The FDA tracks side effects and complaints through its MedWatch system. If a pattern emerges-like a specific generic causing more reports than others-the FDA investigates. They can require changes, issue warnings, or even pull a product off the market. Safety doesn’t end at approval.



Adewumi Gbotemi
January 10, 2026 at 14:31Wow, this is actually super clear. I never knew generics had to pass the same tests as brand names. Makes me feel way better about taking them.
Sean Feng
January 11, 2026 at 18:28The FDA’s just a rubber stamp for pharma. You think they really inspect factories in India? Nah. They just look at the paperwork and call it a day.
Jason Shriner
January 13, 2026 at 11:28Oh great. So now we’re supposed to trust a government agency that can’t even fix the postal service? But sure, let’s pretend this whole system isn’t just corporate theater with a lab coat.
Sam Davies
January 14, 2026 at 07:04It’s fascinating how the FDA’s bioequivalence threshold-80% to 125%-is essentially allowing for a 45% swing in systemic exposure. That’s not precision, that’s pharmaceutical roulette. And yet, somehow, we all live to tell the tale.
Alex Smith
January 16, 2026 at 05:39Interesting how you mention NTI drugs. Most people don’t realize that even a 5% difference in absorption can matter for something like warfarin. That’s why pharmacists often stick you with the same generic-it’s not bureaucracy, it’s harm reduction.
Roshan Joy
January 16, 2026 at 08:16From India here-many of the generics you’re talking about are made in our factories. We take pride in this. The FDA inspections are brutal, and we follow every rule. My cousin works at a plant that got shut down for 6 months over a tiny labeling error. That’s how serious it is.
Michael Patterson
January 16, 2026 at 11:53Look, I’ve been on levothyroxine for 12 years and I’ve switched generics 5 times. Every time I feel like a zombie for two weeks. The FDA says it’s all in my head. But my thyroid doesn’t care about their 80-125% range. It just wants consistency. And if you’re telling me the same drug made in Ohio and made in Hyderabad is interchangeable? I’m not buying it. You’re just repeating what the pharma lobby told you to say.
Matthew Miller
January 18, 2026 at 04:4490% of prescriptions are generic? That’s because doctors are paid kickbacks. The FDA is a joke. You think they care about safety? They care about cost savings and keeping Big Pharma happy. I’ve seen patients die because a generic didn’t work right. And the system just shrugs. This isn’t science-it’s capitalism with a medical license.
Madhav Malhotra
January 19, 2026 at 02:09My uncle in Delhi works at a generic drug plant. He says the inspectors come unannounced, sometimes twice a year. They check everything-even the air quality in the packaging room. People think India is just a cheap factory, but the standards? They’re insane. I’ve seen the reports. It’s not luck-it’s discipline.
Priya Patel
January 20, 2026 at 11:31I switched from brand to generic for my blood pressure med and I almost fainted for a week. I was so scared. But my pharmacist said, ‘Stick with this brand, don’t switch again.’ So I did. And now I’m fine. Don’t be afraid to ask for consistency!
Jennifer Littler
January 20, 2026 at 19:20The bioequivalence criteria are statistically sound, but the assumption of linear pharmacokinetics across populations is problematic. Variability in CYP450 enzyme expression, especially in non-Caucasian populations, isn’t adequately accounted for in the 24-36 subject trials. This is a population-level proxy that doesn’t reflect individual metabolic heterogeneity.
Alfred Schmidt
January 21, 2026 at 14:56WHOA. WHOA. WHOA. Did you just say the FDA inspects 1,500 factories a year?! That’s impossible! They’re understaffed! They’re corrupt! They’re bought off! I read a blog post once that said 70% of generic drugs are contaminated with carcinogens-AND THEY’RE STILL ON THE SHELF!!
Priscilla Kraft
January 22, 2026 at 23:28Thank you for explaining this so clearly! I’ve been on warfarin for years and always worried about switching generics. Now I know it’s okay to ask my pharmacist to keep me on the same one. 🙏
Vincent Clarizio
January 23, 2026 at 16:35Let’s be real. The whole generic drug system is a brilliant scam. The FDA doesn’t approve drugs-they approve paperwork. The real science was done decades ago by the brand companies. Now the generics just copy the formula and sell it for pennies. And we call it progress? It’s just capitalism eating its own tail. The only thing that’s truly ‘innovative’ here is how much money they’re saving while pretending nothing’s changed.
Christian Basel
January 23, 2026 at 18:19Bioequivalence? More like bio-approximation. The 80-125% range is laughable. You can’t claim equivalence if the AUC variance is 45%. That’s not science, that’s a loophole. And don’t get me started on the fact that most of these drugs are made in countries with zero regulatory transparency. It’s a house of cards.