When you pick up a prescription for metformin, lisinopril, or atorvastatin, there’s a 90% chance it’s a generic drug. These medications work just like the brand-name versions you see advertised - same active ingredient, same dose, same effect - but cost up to 85% less. But how exactly are they made? It’s not just copying a pill. The process is highly technical, tightly regulated, and involves science, precision, and years of testing.
Starting with the Original: Reverse Engineering the Brand-Name Drug
The journey of a generic drug begins with the brand-name version, called the Reference Listed Drug (RLD). Manufacturers don’t just look at the pill and guess what’s inside. They use advanced lab tools to break it down - analyzing every component. This includes identifying the exact chemical structure of the active pharmaceutical ingredient (API), the type and amount of inactive ingredients (excipients) like fillers, binders, and coatings, and how the drug is designed to release in the body.This step is critical. A generic drug must match the RLD in strength, dosage form, and how quickly it’s absorbed. If the coating is too thick, the pill might not dissolve fast enough. If the particle size of the API changes, the body might absorb it too slowly or too fast. Even small differences can affect how well the drug works. That’s why manufacturers spend months studying the original product before they even start making their own version.
Designing the Formula: Quality by Design (QbD)
Once the RLD is understood, the next step is designing the generic version using a framework called Quality by Design (QbD). This isn’t just mixing chemicals - it’s engineering a drug to perform exactly like the original.Scientists identify Critical Quality Attributes (CQAs) - the features that matter most to safety and effectiveness. For example, how quickly the drug dissolves in the stomach (dissolution rate) or how stable it is over time. Then they pinpoint the Critical Material Attributes (CMAs) - like the purity of the API or the moisture content of lactose - and Critical Process Parameters (CPPs) - such as mixing time, temperature, and pressure during compression.
Think of it like baking a cake. You can’t just swap flour brands and expect the same result. Same here. If the particle size of the filler changes, the tablet might crumble or take too long to break down. QbD ensures every variable is controlled so the final product performs consistently, batch after batch.
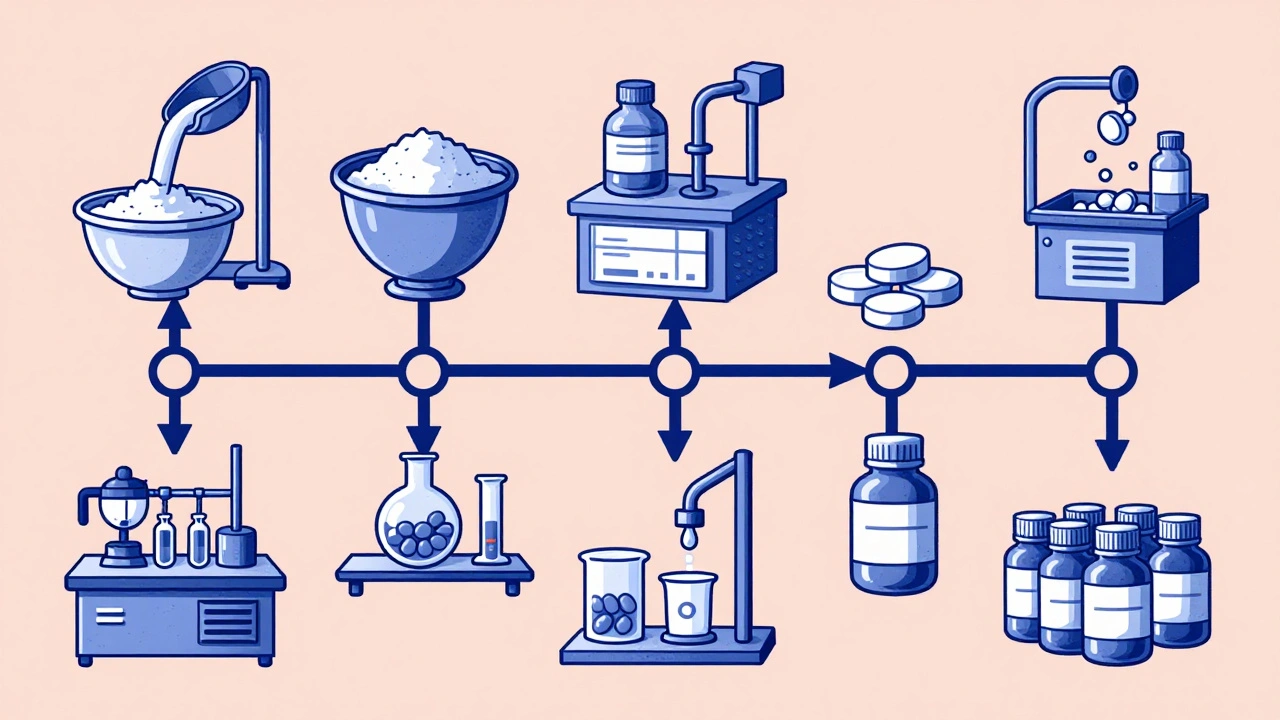
The Seven Steps of Manufacturing
The actual production of generic drugs follows a strict, seven-stage process - each one monitored and documented.- Formulation: The API is blended with excipients like starch, cellulose, or magnesium stearate to create the right consistency and flow properties.
- Mixing and Granulation: The powder blend is turned into granules - tiny clumps - to ensure even distribution of the active ingredient. This prevents hot spots where one pill has too much drug and another too little.
- Drying: Moisture is removed from the granules. Too much water can cause the drug to degrade or the tablet to stick to machinery.
- Compression and Encapsulation: Dry granules are pressed into tablets using high-speed machines. For capsules, the powder is filled into gelatin or plant-based shells. Each tablet must weigh within ±5% to ±7.5% of the target weight, depending on size, as required by FDA standards.
- Coating: Tablets get a thin outer layer. This can mask bitter tastes, protect the drug from stomach acid, or control how fast it releases in the body. Some coatings are designed to release the drug slowly over 12 hours.
- Quality Control: Every batch is tested. Labs check for identity (is it the right drug?), strength (does it have the correct amount of API?), purity (are there harmful impurities?), and dissolution (does it break down properly?). Dissolution testing is especially important - if the drug doesn’t dissolve like the brand-name version, it won’t work the same way.
- Packaging and Labeling: Pills go into bottles or blister packs with labels that match the brand-name drug’s prescribing information. By law, generics can’t look identical to the original - different colors, shapes, or markings are required to avoid confusion and trademark issues.
Where It’s Made: Cleanrooms and CGMP Compliance
Generic drugs aren’t made in basements or small labs. They’re produced in facilities that meet Current Good Manufacturing Practices (CGMP). These are strict rules enforced by the FDA and global regulators.Manufacturing areas are cleanrooms - controlled environments with filtered air, specific temperature (20-25°C), and humidity (45-65% RH). The level of cleanliness depends on the stage: filling capsules might require an ISO Class 5 cleanroom (as clean as a hospital operating room), while packaging might be ISO Class 8.
Every machine, every batch, every step is documented. If a tablet is too light or a dissolution test fails, the entire batch is quarantined. Investigators must find out why - was it a bad batch of raw material? A machine calibration issue? A human error? The fix has to be documented and approved before anything moves forward.
The FDA inspects these facilities regularly. In 2023, 37% of warning letters issued to generic manufacturers cited failures to properly investigate out-of-specification results. That means even small mistakes are taken seriously - and can delay approval or lead to recalls.
The Approval Pathway: The Abbreviated New Drug Application (ANDA)
Here’s where generics differ from brand-name drugs. Brand-name companies spend over a decade and $2.6 billion testing new drugs in clinical trials. Generics don’t need to do that.Instead, they file an Abbreviated New Drug Application (ANDA). The word “abbreviated” means they rely on the FDA’s existing data on the brand-name drug’s safety and effectiveness. Their job? Prove their version is the same.
The key requirement: bioequivalence. This is tested in 24-36 healthy volunteers. Blood samples are taken over hours to measure how much of the drug enters the bloodstream and how fast. The generic’s maximum concentration (Cmax) and total exposure (AUC) must fall within 80%-125% of the brand-name drug’s values - with 90% confidence. If it passes, the FDA says the two drugs are therapeutically equivalent.
The ANDA process takes 17 months on average, but complex drugs - like inhalers, eye drops, or extended-release pills - can take up to three years. And if a patent is still active, the manufacturer might file a Paragraph IV certification, claiming the patent is invalid. That often triggers lawsuits, delaying the generic’s entry by up to 30 months.
Challenges and Real-World Issues
For simple pills like ibuprofen or amoxicillin, making a generic is straightforward. But for complex drugs - think topical creams, nasal sprays, or injectables - it’s a different story.Take Clobetasol Propionate, a powerful steroid cream. One manufacturer spent seven years and $47 million trying to match the original’s skin absorption rate. Even tiny differences in the cream’s texture or pH could change how much drug actually gets into the skin.
Another issue? Raw materials. A pharmaceutical engineer on Reddit shared that changing the particle size of lactose - a common filler - can throw off tablet hardness and dissolution. Suppliers aren’t always consistent. That’s why top manufacturers test every batch of raw material before use.
And while most generics are safe and effective, recalls happen. In 2021, Teva recalled 14 generic drugs due to CGMP violations at its Puerto Rico plant. That doesn’t mean all generics are risky - it means the system needs constant oversight. The FDA inspects over 3,500 facilities worldwide, including many in India and China, where 78% of active ingredients are now made.

Why It Matters: Cost, Access, and Trust
Generic drugs saved the U.S. healthcare system over $1.7 trillion in the last decade. Without them, millions of people couldn’t afford their medications. A generic version of Sovaldi, a hepatitis C drug, dropped the price from $84,000 to $28,000 per course - a life-changing difference.Yet, some patients worry: “Is my generic really the same?” The answer, backed by data, is yes. A 2023 survey found 89% of pharmacists have high confidence in generic quality. Only 3% reported any meaningful difference in patient outcomes.
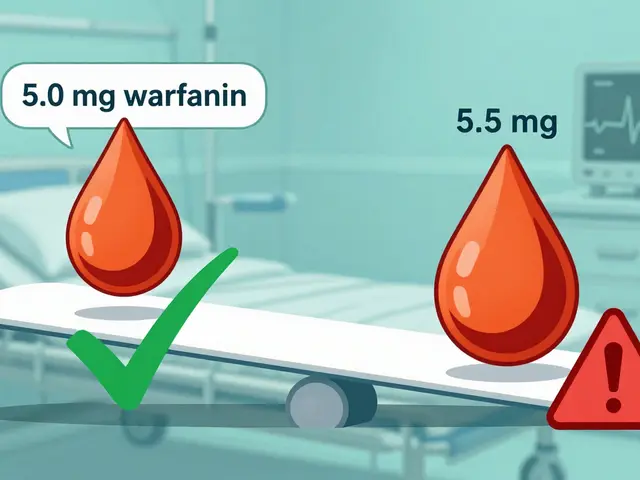
For drugs with a narrow therapeutic index - like warfarin or lithium - even small differences matter. That’s why doctors sometimes stick with one brand. But studies show that when patients switch between generic manufacturers, there’s no consistent pattern of harm. The FDA requires all generics to meet the same strict standards, no matter who makes them.
The Future: AI, Continuous Manufacturing, and Global Shifts
The industry is changing. The FDA now approves continuous manufacturing - where drugs are made in one seamless flow instead of in batches. Vertex’s cystic fibrosis drug, made this way, achieved 99.98% batch acceptance, compared to 95% with traditional methods.AI is being used for quality control. Pfizer’s pilot program cut visual inspection errors by 40% by using machine learning to spot defects in pills faster than human eyes.
And as more complex drugs go generic - like biologics and inhalers - the focus is shifting to better testing methods. The FDA is working on new guidelines for nasal sprays, eye drops, and topical products that can’t be fully measured with standard blood tests.
Despite challenges, the future of generics is strong. With $75 billion in branded drugs set to lose patent protection by 2027, demand for affordable alternatives will only grow. The system isn’t perfect - but it’s working. Millions rely on it every day.
Are generic drugs as safe as brand-name drugs?
Yes. The FDA requires generic drugs to have the same active ingredient, strength, dosage form, and route of administration as the brand-name version. They must also prove bioequivalence - meaning they work the same way in the body. The same manufacturing standards (CGMP) apply to both. The only differences are in inactive ingredients, color, shape, or packaging - none of which affect safety or effectiveness.
Why do generic pills look different from brand-name ones?
U.S. trademark laws prevent generic manufacturers from copying the exact appearance of brand-name drugs. So generics have different colors, shapes, or markings. This avoids confusion and protects brand trademarks. But the active ingredient and how it works are identical. If you’re unsure, check the drug’s name on the label - it’s always listed clearly.
Can different generic brands of the same drug work differently?
All FDA-approved generics must meet the same bioequivalence standards. In most cases, switching between generic brands has no effect. However, for drugs with a narrow therapeutic index - like warfarin or thyroid medication - small differences in how the drug is absorbed can matter. If you notice changes in how you feel after switching generics, talk to your doctor. They may recommend sticking with one manufacturer.
How long does it take to make a generic drug?
For simple pills, the entire process - from reverse engineering to FDA approval - takes about 3-4 years and costs $5-10 million. Complex drugs like inhalers or extended-release formulations can take 5-7 years and cost over $50 million. The FDA’s review of the application averages 17 months, but can stretch to 36 months for complicated cases.
Why are most generic drugs made in India and China?
India and China produce the majority of the world’s active pharmaceutical ingredients because of lower labor and production costs. The FDA inspects facilities in both countries regularly - over 3,500 global sites are under review. While concerns about quality exist, the FDA’s standards apply equally to all manufacturers, regardless of location. Most generic drugs sold in the U.S. are made under FDA-approved conditions, even if the raw materials come from overseas.
What’s the difference between generic and biosimilar drugs?
Generic drugs are copies of small-molecule chemical drugs, like aspirin or metformin. Biosimilars are copies of large, complex biological drugs - like insulin or cancer treatments made from living cells. Because biological drugs are made from living organisms, biosimilars can’t be exact copies. They must be shown to be “highly similar” with no clinically meaningful differences. The approval process for biosimilars is more complex and requires more testing than for traditional generics.

Scott Butler
December 13, 2025 at 23:11This is why America needs to stop outsourcing our pharma to China and India. These facilities are barely inspected, and now we're all guinea pigs for cheap generics. The FDA's 'approval' is a joke. I'd rather pay more for a pill made in the USA than risk my life because some factory in Mumbai cut corners.
Emma Sbarge
December 15, 2025 at 19:19The manufacturing process described here is actually fascinating. The level of precision required - down to particle size and moisture content - is insane. It's not just copying a pill. It's reverse-engineering biology with a microscope and a spreadsheet. People think generics are cheap because they're lazy, but they're actually the result of incredibly rigorous science.
Ronan Lansbury
December 16, 2025 at 16:53Let me guess - the FDA approves these because Big Pharma owns them. You think these cleanrooms are clean? Ha. The real story is that 80% of API comes from China, and the FDA hasn't set foot in half those plants since 2018. This is a controlled illusion. They want you to believe it's safe so you'll keep taking it while they profit off the brand-name patents they still hold.
Jennifer Taylor
December 18, 2025 at 09:20I switched my blood pressure med to generic and felt like a zombie for two weeks. My heart was racing, I couldn't sleep, I was sweating like I'd run a marathon in a sauna. I went back to the brand and BOOM - normal again. So yeah, they're NOT the same. And no one wants to admit it because the system is rigged. I'm not crazy, I'm just one of the 3% they ignore. #GenericDangers
Rawlson King
December 19, 2025 at 13:16The QbD framework is actually brilliant. Most people don’t realize that pharmaceutical manufacturing is closer to aerospace engineering than it is to baking cookies. Every variable is mapped, every deviation logged, every batch traceable. It’s not magic - it’s math, physics, and obsessive documentation. The fact that this works at scale is a triumph of systems engineering.
Michael Gardner
December 21, 2025 at 10:57You say generics are just as good? Prove it. Where’s the long-term, double-blind study comparing 10-year outcomes between brand and generic metformin users? You can’t. Because they don’t exist. The bioequivalence window of 80-125% means a generic could be 25% weaker or 25% stronger - that’s not equivalence, that’s a lottery ticket.
Willie Onst
December 23, 2025 at 03:49Honestly? This post made me appreciate my meds way more. I used to think generics were just ‘cheaper versions’ - now I see them as this quiet miracle of science and regulation. Millions of people get life-saving drugs because of this system. It’s not glamorous, but it’s one of the most important things our government gets right. Thanks for explaining it so clearly.
Shelby Ume
December 24, 2025 at 17:40I work in clinical pharmacy, and I can tell you: for 98% of patients, generics are indistinguishable from brand-name drugs. The exceptions - warfarin, levothyroxine, some seizure meds - are well-documented, and prescribers know to flag them. The panic around generics is mostly anecdotal and amplified by fear, not data. Stick with the science, not the Reddit horror stories.
Jade Hovet
December 26, 2025 at 01:12OMG this is so cool!! 😍 I had no idea how much science went into making a simple pill!! Like, the coating? The particle size?? I thought it was just powdered stuff in a mold 🤯 My grandma takes 7 generics and I just told her all this - she’s gonna feel like a superhero now!! 💪💊 #PharmaMagic
John Fred
December 27, 2025 at 14:11Bioequivalence is a statistical construct, not a biological guarantee. Cmax and AUC windows are meaningless when you're dealing with pharmacokinetic outliers. Add in inter-patient variability, gut microbiome differences, and food interactions - and suddenly that 80-125% window becomes a minefield. This isn’t just chemistry - it’s chaos theory in a tablet.
Harriet Wollaston
December 28, 2025 at 04:25I just want to say thank you for writing this. My dad’s on a generic for his heart condition, and I was terrified he was getting a substandard version. Reading this made me feel so much better. It’s easy to fear what you don’t understand - but this is a beautiful example of how science, regulation, and compassion can come together to save lives.
Hamza Laassili
December 28, 2025 at 19:02I saw this on the news - one of those generic insulin pens? Totally fake. The label said ‘Made in USA’ but the barcode traced back to a warehouse in Guangzhou. And the FDA? They didn’t catch it for 18 months. They’re asleep at the wheel. We’re being lied to. #GenericScam #ChinaControlsOurMedicine
Lara Tobin
December 30, 2025 at 07:54I’m so glad someone finally explained this. I’ve been nervous about switching to generics for years, but this made me feel like it’s okay. I’m not alone in worrying, and it’s good to know there’s real science behind it. Thank you for taking the time to write this - it means a lot.