When you pick up a prescription for insulin or a biologic drug like Humira, you might not realize the pharmacy could swap it out for a different version without your doctor’s approval. That’s not a mistake-it’s called interchangeability, and it’s a unique feature of how the U.S. handles biosimilars. Unlike generic pills, which are chemically identical to their brand-name counterparts, biosimilars are made from living cells. They’re incredibly complex, and getting them approved as interchangeable isn’t just about matching the original drug-it’s about proving you can switch back and forth between them without risking your health.
What Makes a Biosimilar Interchangeable?
Not all biosimilars are created equal. The FDA approves two types: biosimilars and interchangeable biosimilars. A regular biosimilar must show it’s highly similar to the reference biologic in structure, function, safety, and effectiveness. But to be labeled interchangeable, a drug has to go further. It must prove that switching between the reference product and the biosimilar-multiple times-won’t increase risks or reduce effectiveness. That means clinical studies where patients are switched from the original drug to the biosimilar, then back again, and sometimes even a third time. These aren’t short-term tests. They’re designed to catch subtle changes in immune response or side effects that might show up after repeated switches. The FDA requires this because biologics are made in living systems-like yeast or hamster cells-and tiny differences in manufacturing can change how the body reacts. The first interchangeable biosimilar approved was Semglee, an insulin glargine product, in July 2021. Before that, patients had to get a new prescription every time they switched. Now, in many places, a pharmacist can swap it out automatically. Then in August 2023, Cyltezo became the first interchangeable biosimilar for adalimumab (Humira), a drug used for rheumatoid arthritis, psoriasis, and Crohn’s disease. That opened the door for millions of patients.How Is This Different from Generic Drugs?
Think of generic drugs like aspirin or metformin. They’re small molecules, made in labs with exact chemical formulas. If you make them under the same conditions, they’re virtually identical. That’s why pharmacists can swap them without hesitation. Biosimilars? They’re more like a handmade quilt than a factory-printed T-shirt. Even if two companies use the same blueprint, the living cells they grow them in might produce slightly different proteins. That’s why the FDA treats them differently. A generic drug just needs to prove it’s bioequivalent-same absorption, same blood levels. An interchangeable biosimilar has to prove you can switch back and forth safely, like swapping out two different brands of car battery and never noticing a difference in how the engine runs. As of November 2023, the FDA has approved 41 biosimilars, but only 10 have the interchangeable label. That gap shows how tough the bar is.State Laws Make It a Patchwork
Even if the FDA says a biosimilar is interchangeable, that doesn’t mean every pharmacy can swap it automatically. Each state has its own rules. Forty states, including Arizona and California, let pharmacists substitute without asking the doctor-so long as the product is designated interchangeable. But six states, like Arkansas and Mississippi, only allow substitution if it saves the patient money. Four states-Alabama, Indiana, South Carolina, and Washington-require the prescriber’s permission every time. This creates real headaches for pharmacists. One community pharmacist in Texas told a 2023 survey they spent nearly nine hours a year just keeping up with state rules. National chains have to program their systems to check the patient’s address before deciding whether substitution is allowed. Independent pharmacies often don’t have the staff or software to handle it smoothly. And it’s not just about legality. Insurance plans also play a role. About 78% of commercial health plans require automatic substitution for interchangeable biosimilars when state law allows it. So even if your doctor writes "dispense as written," your insurer might override it unless you specifically opt out.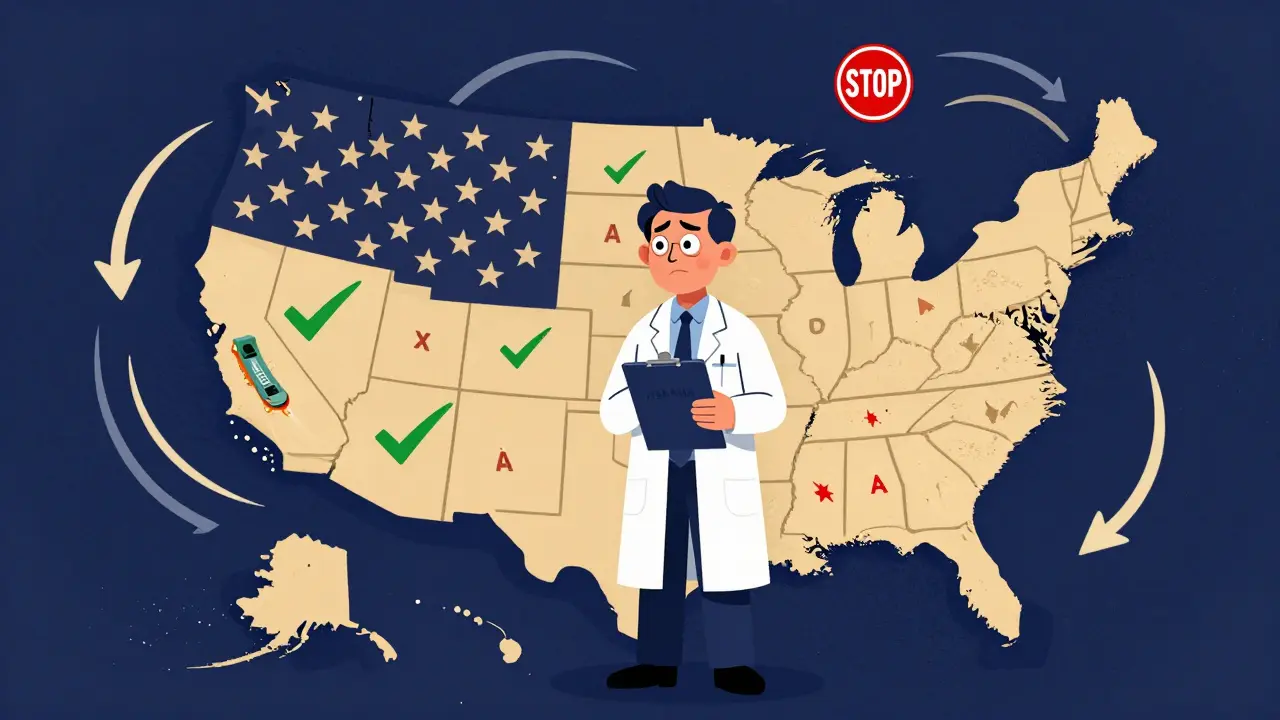
What Patients Are Saying
For many, interchangeability means savings. One patient on the Psoriasis Foundation forum said switching from Humira to Hyrimoz saved them $800 a month-with no change in how they felt. That’s huge for people on expensive biologics that can cost over $20,000 a year. But not everyone has smooth transitions. Another patient reported an allergic reaction after their pharmacy automatically switched them from Humira to Hadlima. Turns out, they were sensitive to a stabilizer in the new formulation. The reaction wasn’t caused by the active ingredient-it was an excipient, something added to keep the drug stable. That’s why the FDA says all biosimilars, even interchangeable ones, must list every ingredient. But many patients don’t know to check. A 2022 survey by the National Psoriasis Foundation found 63% of patients were happy with their biosimilar, but nearly 30% were upset they weren’t told about the switch. Transparency matters. In Arizona, pharmacists must notify patients in writing and send a record to the doctor within five days. In other states, no such rule exists.Why Experts Are Divided
Supporters say interchangeability is the key to cutting costs in the $300 billion biologics market. A 2022 RAND Corporation study found biosimilars typically cost 15-30% less than the original. With insulin alone, the first interchangeable version, Semglee, grabbed 17.3% of the market within six months-much faster than non-interchangeable versions. But critics worry about unintended consequences. Dr. Kevin Winthrop, a professor at Oregon Health & Science University, pointed to a study showing psoriasis patients switched to biosimilars were 20% more likely to stop treatment entirely. Was it because the drug didn’t work? Or because they didn’t trust the switch? No one knows for sure. There’s also a push in Congress to eliminate the switching studies entirely. The Biosimilar Red Tape Elimination Act, introduced in 2022, would make every FDA-approved biosimilar automatically interchangeable. Supporters say it’s unnecessary bureaucracy. Opponents, including PhRMA, argue it could put patients at risk by skipping critical safety data. The FDA’s position is clear: all approved biosimilars are safe and effective. Interchangeability doesn’t mean "better"-it just means "switchable without a doctor’s note." But the public doesn’t always hear that distinction.
What Pharmacists Need to Know
If you’re a pharmacist, you’re on the front lines. You need to know:- Is the biosimilar FDA-designated as interchangeable?
- What does your state law say about substitution?
- Does the patient’s insurance require substitution?
- Did the prescriber write "dispense as written" or use a DAW code?
- Are you required to notify the patient and the doctor?
The Future of Interchangeability
By 2025, 70% of the top 20 biologic drugs in the U.S. will have biosimilar competitors. That’s a massive shift. Insulin, Humira, Enbrel, Remicade-these are all in play. Interchangeable versions will likely become the norm, not the exception. But the path forward isn’t smooth. State laws are inconsistent. Patient education is lacking. Pharmacists are overwhelmed. And Congress is debating whether to simplify the rules-or make them even more complex. One thing is certain: if you’re on a biologic, you need to know your options. Ask your doctor: "Is there a biosimilar available?" Ask your pharmacist: "Was this switched?" And always check the label. Your health depends on knowing what’s in the bottle.Can any biosimilar be automatically substituted at the pharmacy?
No. Only biosimilars that have received an "interchangeable" designation from the FDA can be substituted automatically. Most biosimilars approved in the U.S. are not interchangeable. Pharmacists can only swap them for the original drug if they’re labeled interchangeable and if state law allows it.
Are interchangeable biosimilars safer or more effective than regular biosimilars?
No. Both interchangeable and non-interchangeable biosimilars must meet the same strict FDA standards for safety, purity, and potency. The only difference is that interchangeable biosimilars have passed extra studies proving you can switch back and forth between them and the original drug without increased risk.
Can a pharmacist switch me from one biosimilar to another?
No. FDA interchangeability only applies to switching between a reference biologic and its interchangeable biosimilar. It does not allow substitution between two different biosimilars of the same reference product. For example, if you’re on Cyltezo (an interchangeable Humira biosimilar), your pharmacist can’t switch you to another Humira biosimilar like Hyrimoz without your doctor’s approval.
Why do some states require doctor approval for substitution?
Some states are cautious because of concerns about patient safety, lack of long-term data on multiple switches, or pressure from pharmaceutical companies. States like Alabama, Indiana, South Carolina, and Washington require prescriber consent to ensure the patient and doctor are aware of any change in treatment, especially for chronic conditions like rheumatoid arthritis or Crohn’s disease.
How can I find out if my medication was switched to a biosimilar?
Check the prescription label-it must list the manufacturer’s name. You can also ask your pharmacist directly. Some states require pharmacists to notify you in writing when a substitution occurs. If you notice new side effects or a change in how the drug works, contact your doctor immediately and ask whether a switch happened.
Do insurance companies force substitution to interchangeable biosimilars?
Yes. About 78% of commercial health plans require automatic substitution for interchangeable biosimilars when state law permits it. Even if your doctor writes "dispense as written," your insurer may override it unless you formally opt out. Always check your plan’s formulary and ask your pharmacist about substitution policies.




kate jones
January 31, 2026 at 09:40Interchangeability isn't just a regulatory checkbox-it's a clinical protocol wrapped in bureaucracy. The FDA’s requirement for multiple switch studies isn’t overkill; it’s the only way to detect immunogenicity cascades that might emerge after the third or fourth switch. We’ve seen this with monoclonal antibodies in rheumatology: subtle changes in glycosylation patterns alter Fc receptor binding, and that’s not something you catch in a 12-week trial. The real issue isn’t safety-it’s transparency. Patients need to know not just that a switch occurred, but which excipients changed, because those are often the culprits behind hypersensitivity reactions.
And pharmacists? They’re drowning in state-by-state DAW codes and payer mandates. No one’s accounting for the cognitive load of checking 50 different rules per prescription. This system isn’t sustainable unless we standardize the data exchange between EHRs, pharmacies, and insurers.
Also, let’s not pretend biosimilars are ‘just generics.’ They’re living products. The cell line, the bioreactor temperature, the purification buffer-all of it matters. Two interchangeable biosimilars aren’t identical. They’re functionally equivalent under controlled conditions. That’s not semantics-it’s pharmacology.
And yes, the 30% non-adherence spike after switching? That’s likely psychosocial, not pharmacological. Patients don’t trust what they don’t understand. We need patient-facing decision aids, not just pharmacy notices.
Bottom line: interchangeability is a tool. Used right, it saves lives and money. Used poorly, it erodes trust. We’re not close to getting it right yet.
Lisa McCluskey
January 31, 2026 at 09:48My dad switched from Humira to Cyltezo last year. No issues. Saved him $1,200 a month. He didn’t even notice until I checked the bottle.
Simple as that.
Marc Bains
February 1, 2026 at 05:09For anyone outside the U.S. reading this: this is why your country’s healthcare system works better. We’ve turned a scientific breakthrough into a legal maze. Pharmacists are now de facto clinicians, forced to interpret 50 different state laws while juggling insurance rules and prescriber preferences. Meanwhile, patients get a pill they didn’t ask for and a bill they didn’t expect.
We need federal baseline standards. Not because states are bad-but because patients shouldn’t get different care based on their ZIP code. This isn’t innovation. It’s fragmentation.
And if Congress passes that Red Tape Elimination Act without patient safeguards, we’re going to regret it. Not because biosimilars are unsafe-but because we’ll have normalized substitution without consent. That’s not efficiency. That’s erasure.
owori patrick
February 2, 2026 at 11:22As a Nigerian pharmacist working with global supply chains, I find this fascinating. In my country, we don’t even have biosimilars yet, let alone interchangeability rules. But I’ve seen how confusion around generic substitutions leads to mistrust. If the U.S. can get this right-with education, transparency, and clear labeling-it could be a model for low- and middle-income countries when they scale up access to biologics.
Don’t let bureaucracy kill the opportunity. Teach patients. Train pharmacists. Simplify the system. We’re talking about insulin here-people die without it. This isn’t about profit. It’s about survival.
Claire Wiltshire
February 3, 2026 at 09:13I work in a community pharmacy in Ohio. We’ve been substituting Semglee since late 2021. We notify every patient in writing, update the EHR, and send a note to the prescriber. It takes 3 minutes per script.
Most patients are thrilled. One woman cried because her copay dropped from $500 to $75.
But we’ve had three cases where patients came back saying their pain flared up. Turns out, two were sensitive to polysorbate 80 in the new formulation. The original had a different stabilizer.
So yes-interchangeable doesn’t mean identical. And yes-we need better labeling. But the system works when we do our job right.
Don’t blame the pharmacist. Blame the lack of federal standardization and the insurers who force switches without telling anyone.
Eliana Botelho
February 5, 2026 at 07:57Okay but like, why are we even pretending this is science? It’s all about money. Big Pharma doesn’t want to lose their $20k/year monopoly, so they lobbied to make the bar for interchangeability so high that only 10 out of 41 biosimilars cleared it. Meanwhile, patients are getting switched like they’re trading sneakers.
And don’t even get me started on state laws. Alabama requires a doctor’s note? Bro, that’s not safety, that’s just corporate lobbying dressed up as caution. The real reason? Insurance companies pay pharmacists a kickback for switching. It’s a cash grab disguised as cost-saving.
And don’t tell me about ‘clinical studies.’ If the FDA approved the original biosimilar, why do we need a whole new trial just to let a pharmacist swap it? It’s nonsense. Let’s just make all biosimilars interchangeable and stop pretending we’re protecting patients when we’re really protecting profits.
Also, I switched to a biosimilar and my psoriasis got worse. My doctor said it was ‘likely coincidental.’ Yeah right. I’m not stupid. They just don’t care.
Wake up. This isn’t medicine. It’s capitalism with a stethoscope.
Russ Kelemen
February 6, 2026 at 01:21Let’s reframe this: interchangeability isn’t about drugs. It’s about trust.
Patients trust their doctors to choose the right treatment. They trust pharmacists to fill it correctly. They trust insurers to not screw them on cost.
Right now, the system is breaking that trust at every level. A patient gets switched without knowing why. They feel like a lab rat. Their doctor didn’t approve it. Their insurer forced it. The label doesn’t explain the excipient change.
We’re not failing because of science. We’re failing because we’re not communicating.
Every time a biosimilar is dispensed, there should be a QR code on the bottle that links to a 90-second video explaining: what changed, why it’s safe, what to watch for, and who to call if something feels off.
That’s not expensive. That’s human.
And if we do that? Interchangeability becomes a win for everyone. Not just the bottom line.
Rob Webber
February 6, 2026 at 13:18So let me get this straight. The FDA says a biosimilar is interchangeable, but then some states say ‘nope, you need a doctor’s note’ and others say ‘only if it saves money’ and insurers override everything anyway? This isn’t healthcare. This is a glitch in the Matrix.
And now we’re supposed to believe that switching back and forth between two biologics made in hamster cells is ‘safe’? You’re telling me a protein folded differently because of a 0.5°C temperature fluctuation in a bioreactor in New Jersey is functionally identical to one made in Germany? Bullshit.
They’re not interchangeable. They’re interchangeable in the eyes of a regulator who’s been lobbied into submission.
And don’t even get me started on the fact that no one’s tracking long-term outcomes. We’re the guinea pigs. And you know what? I’m not okay with that.
Natasha Plebani
February 6, 2026 at 16:31Interchangeability, as a concept, reveals a deeper epistemological tension in modern pharmacology: the illusion of equivalence.
Biologics are not molecules. They are emergent phenomena-products of living systems, shaped by culture, environment, and process. To treat them as if they were aspirin is to commit a category error.
The FDA’s requirement for multiple switch studies is not merely a regulatory hurdle-it is an acknowledgment that identity in biological systems is not reducible to chemical structure. It is performative. It is relational. It is context-dependent.
When we say ‘interchangeable,’ we are not saying ‘identical.’ We are saying ‘sufficiently similar under controlled conditions to not trigger a measurable clinical deviation.’
But what is ‘measurable’? And whose deviation counts?
Is a 2% increase in anti-drug antibody titers clinically relevant? Or is it a statistical artifact masked by the noise of chronic inflammation?
We have built a system that demands certainty where none exists. And then we call it science.
Perhaps the real question isn’t whether biosimilars can be switched-but whether we should be switching at all, without consent, without context, without humility.
Darren Gormley
February 7, 2026 at 01:38😂😂😂 oh my god this is peak American healthcare. We have 50 different state laws about swapping insulin because someone in Alabama is scared of hamster cells. Meanwhile, in Canada they just say ‘take the cheaper one’ and move on. No forms. No notifications. No drama.
And yet we’re the ones who think we’re ‘innovating.’
Also, the fact that 78% of insurers force substitution? That’s not ‘cost-saving’-that’s corporate coercion. You’re not saving money. You’re shifting risk onto patients who don’t know what’s in the vial.
And don’t even get me started on the fact that you can’t switch between two biosimilars. So if you’re on Cyltezo, and your pharmacy runs out? Tough. You need a new script. Because apparently, two biosimilars are ‘different’ but one biosimilar and the original are ‘the same.’ 🤡
Someone please explain this to me like I’m a 5-year-old. Or better yet, someone please fix it.
Mike Rose
February 7, 2026 at 21:12bro why are we even talking about this. just give me the cheap one. i dont care if its made by hamsters or robots. if it works and costs less, im good.
also why do i need a 10 page notice just to get my insulin? i just want to not go broke.
Katie and Nathan Milburn
February 8, 2026 at 18:19As someone who has managed biologic therapies for over 15 years in academic practice, I can say with certainty that the data supporting interchangeability is robust-but the implementation is catastrophic. The problem is not the science. It is the lack of standardized communication protocols, the absence of real-time EHR integration, and the failure to educate both prescribers and patients. We have the tools. We have the evidence. What we lack is systemic will.
Interchangeability, properly executed, reduces disparities in access. But when it’s weaponized by insurers and fragmented by state law, it becomes a source of inequity. The solution is not to eliminate the standards. It is to enforce them uniformly-with patient consent, transparent labeling, and mandatory reporting of adverse events tied to substitution.
This isn’t about politics. It’s about professionalism.
kate jones
February 9, 2026 at 18:04Just to clarify something I saw in another comment: switching between two different biosimilars (e.g., Cyltezo → Hyrimoz) isn’t allowed because interchangeability is defined relative to the reference product-not between biosimilars. That’s not a flaw. It’s a safeguard. Each biosimilar has its own manufacturing footprint. Even if both are interchangeable with Humira, they’re not necessarily interchangeable with each other. The FDA didn’t test that. So pharmacists aren’t allowed to swap. That’s not bureaucracy. That’s pharmacovigilance.
And yes, the excipient issue is real. One patient had anaphylaxis to polysorbate 80 in Hadlima. The same patient had no reaction to Humira or Cyltezo. The active ingredient was identical. The stabilizer wasn’t. That’s why the FDA requires full ingredient disclosure. But no one reads the label. That’s the real failure.