When a patient can’t take a standard pill because they’re allergic to dyes, need a tiny dose for a child, or can’t swallow tablets, customized medications are often the only solution. These aren’t mass-produced drugs - they’re made one at a time by pharmacists who mix ingredients to match exact patient needs. But this personalization comes with serious risks. One wrong measurement, one mislabeled bottle, and a life-saving medicine can turn deadly. Between 2018 and 2022, the FDA reported 27 fentanyl overdoses from compounded medications because labels didn’t clearly state concentration per mL. That’s not a rare mistake - it’s a preventable one.
Why Compounding Errors Happen
Compounding isn’t like factory drug production. FDA-approved pills go through clinical trials with thousands of people. Compounded meds? They don’t. That means every batch relies entirely on the pharmacist’s skill, training, and systems. Common mistakes include:- Wrong ingredient - mixing up potassium chloride for potassium citrate
- Incorrect concentration - labeling 10 mg/mL as 10 mg per container
- Contamination - using dirty equipment or working in unclean air
- Calculation errors - miscalculating doses for a 12-pound infant
- Poor labeling - unclear units, missing beyond-use dates
A 2021 study found that 3-15% of compounded preparations had significant deviations from the intended formula. That’s not a small number. For a child with epilepsy needing 0.5 mg of a drug daily, a 10% error could mean a seizure - or worse.
The Three Core Rules to Prevent Errors
There’s no magic tool or single fix. Preventing errors comes down to three non-negotiable practices backed by USP standards and real-world results.1. Dual Verification for Every Step
No single pharmacist should calculate, measure, or label a compounded medication alone. The American Society of Health-System Pharmacists (ASHP) requires a second qualified professional to independently check every calculation, ingredient, and final product. This isn’t optional. It’s the baseline.One pharmacist measures 50 mg of active ingredient. The second pharmacist uses a calibrated scale and re-weighs it. Then they cross-check the lot number on the ingredient container against the prescription. Finally, they verify the final label says “5 mg/mL” - not “5 mg per vial.”
Studies show this dual-check system cuts errors by more than 60%. A 2022 study in the Journal of the American Pharmacists Association found that pharmacies using mandatory dual verification had 40% fewer errors than those that didn’t.
2. Follow USP <795> and <797> Exactly
The United States Pharmacopeia (USP) sets the gold standard. USP <795> covers non-sterile compounding - things like oral liquids, creams, or suppositories. USP <797> covers sterile compounds - injections, IV bags, eye drops. These aren’t suggestions. They’re rules.For sterile compounding, USP <797> requires:
- Working in an ISO Class 5 cleanroom (like a hospital operating room)
- Staff wearing sterile gowns, gloves, masks
- Media fill testing twice a year to prove sterile technique
- Environmental monitoring for particles and microbes daily
For non-sterile, USP <795> requires:
- A dedicated clean area with ISO Class 8 air quality
- Separate tools for each formulation
- Documentation of every ingredient’s source and lot number
Dr. Linda Tyler, chair of the USP Compounding Expert Committee, says adherence to these standards reduces errors by at least 60%. Facilities that skip them are playing Russian roulette with patient safety.
3. Use Technology - Not as a Crutch, But as a Guardrail
Software like PharmScript and Compounding.io doesn’t replace human judgment - it reinforces it. These systems:- Automatically flag impossible doses (e.g., 500 mg for a 5-year-old)
- Verify ingredient compatibility (e.g., don’t mix this drug with that base)
- Generate electronic batch records with timestamps and signatures
- Integrate with barcode scanners to confirm ingredient identity
A 2022 pilot study of AI-powered verification tools like CompoundingGuard AI showed an 87% drop in calculation errors. Even basic barcode scanning for ingredients cut identification mistakes by 92% in a University of Tennessee case study.
But tech only works if staff use it correctly. A pharmacy that disables alerts or skips scans is worse off than one with no software at all.
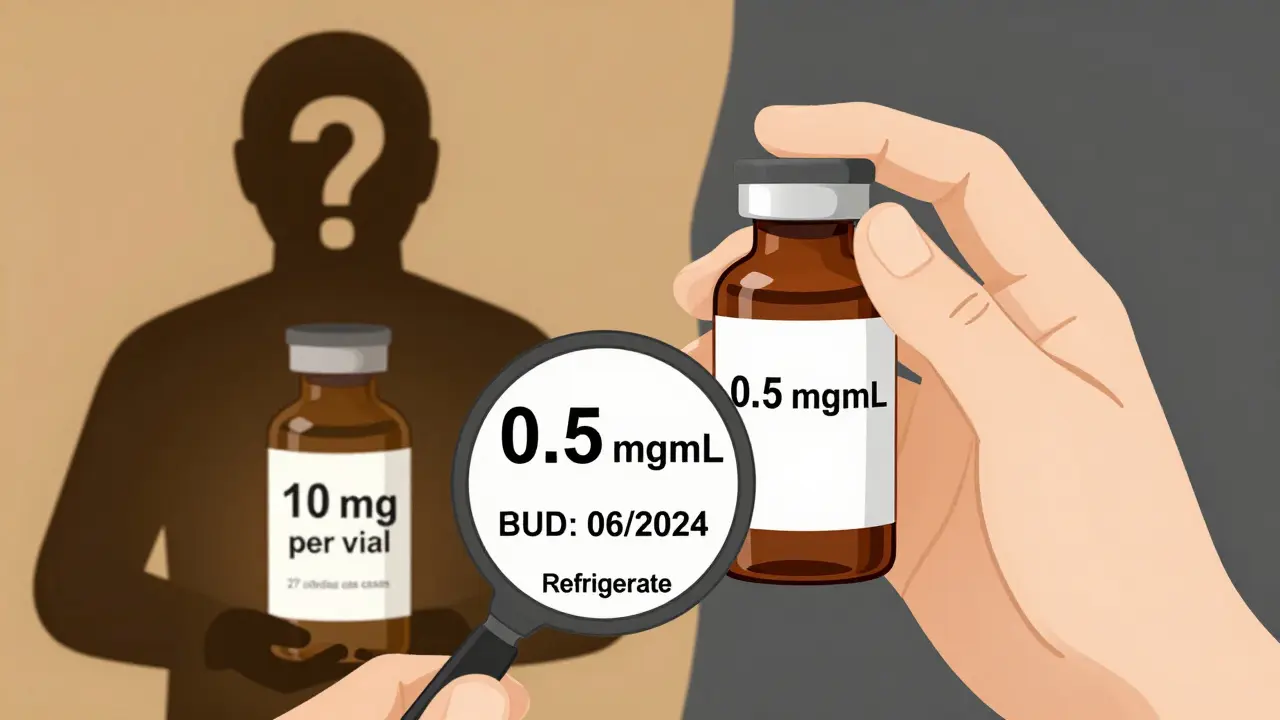
Labeling: The Silent Killer
One of the most dangerous gaps in compounding is labeling. In 2023, the FDA released draft guidance requiring all compounded labels to use clear concentration units: “mg/mL” - not “mg per container” or “per vial.” Why? Because in 27 overdose cases between 2018-2022, prescribers and nurses misread the label. They thought a vial contained 10 mg total - when it actually had 10 mg per mL, and the vial held 5 mL. That’s 50 mg - five times the intended dose.Proper labeling means:
- Concentration always in “mg/mL” or “units/mL”
- Beyond-use date (BUD) clearly printed
- Storage instructions (e.g., “Refrigerate” or “Use within 4 hours”)
- Batch number and pharmacist initials
For non-sterile compounds, BUDs typically range from 30 to 180 days. For sterile, it’s 3 hours to 45 days - depending on how it was made and stored. Never guess a BUD. Test it. Or follow published stability data from peer-reviewed studies.

Training Isn’t a One-Time Event
A pharmacist who learned compounding in 2015 isn’t prepared for today’s standards. The Joint Commission found that 18% of medication error sentinel events between 2019-2022 were rooted in inadequate training.Effective training includes:
- 40+ hours of initial hands-on compounding education
- Quarterly competency assessments - not just quizzes, but live tests
- Annual 8-12 hours of continuing education on new USP rules
- Drills for contamination events and mislabeling scenarios
Dr. Henry Cohen, past president of the International Academy of Compounding Pharmacists, says: “The single most effective error prevention strategy is rigorous staff training with mandatory competency assessments conducted quarterly.”
Accreditation: The Gold Standard
The Pharmacy Compounding Accreditation Board (PCAB) sets the highest bar. Only 18% of U.S. compounding pharmacies are PCAB-accredited as of 2023 - up from 8% in 2018. Why does it matter?PCAB requires:
- Strict adherence to USP <795> and <797>
- 95% accuracy in dose verification tests
- Complete documentation of every batch
- Regular environmental monitoring
- Staff training logs and competency records
Dr. Robert Smith of Harvard Medical School found that error rates in PCAB-accredited pharmacies were as low as 2%. In non-accredited ones? As high as 25%. That’s not a difference in skill - it’s a difference in systems.
Getting accredited takes 12-18 months and costs $15,000-$25,000. But the cost of one preventable death? That’s priceless.
What Patients Should Ask
You don’t need to be a pharmacist to protect yourself. If you’re getting a compounded medication, ask:- “Is your pharmacy PCAB-accredited?”
- “Do you use dual verification for every prescription?”
- “Can I see the label? Does it say concentration in mg/mL?”
- “What’s the beyond-use date? How should I store it?”
Parents of children on compounded thyroid meds, or elderly patients on custom pain formulas, should never assume safety. Ask. Push. Document. Your life depends on it.
The Bigger Picture
Compounding isn’t going away. Demand is growing - 7.8% annual growth through 2030, according to Grand View Research. More patients need allergen-free, pediatric, or alternative-formula meds. But without standardization, the system will keep failing.The FDA’s 2023 Strategic Plan aims to cut compounding-related adverse events by 50% in five years. That means stricter oversight, mandatory reporting, and likely national minimum standards. Until then, the burden falls on pharmacists - and the patients who trust them.
Preventing errors isn’t about perfection. It’s about layers: training, verification, technology, labeling, and accountability. Skip one layer, and the whole system cracks.
What’s the biggest cause of compounding errors?
The biggest cause is skipping the dual-check system. Many errors happen because one person does the entire process - measuring, calculating, labeling - without a second set of eyes. Studies show that requiring a second pharmacist to independently verify every step reduces errors by over 60%. It’s not about distrust - it’s about human limits. Everyone makes mistakes. Systems prevent them.
Can I trust a compounding pharmacy that isn’t PCAB-accredited?
You can, but you shouldn’t assume it’s safe. PCAB accreditation is the only nationally recognized standard that verifies a pharmacy follows USP <795> and <797> rigorously. Non-accredited pharmacies can still be safe - but without independent verification, there’s no way to know. Ask if they use dual verification, have cleanroom standards, and maintain full batch records. If they can’t answer clearly, find another pharmacy.
Why are beyond-use dates so important?
Beyond-use dates (BUDs) tell you how long a compounded medication stays safe and effective. Unlike factory drugs with expiration dates based on years of testing, compounded meds rely on stability data from lab studies. A cream might last 180 days if refrigerated. A sterile IV bag might only last 48 hours. Using a compound past its BUD can mean reduced potency - or dangerous bacterial growth. Never use a compounded med past its printed date.
How do I know if my compounded medication is labeled correctly?
Look for three things: concentration in mg/mL (or units/mL), the beyond-use date, and storage instructions. If it says “10 mg per vial” instead of “10 mg/mL,” that’s dangerous - you don’t know how much liquid is in the vial. Also check for the pharmacist’s initials and batch number. If any of this is missing, call the pharmacy and ask for clarification before taking it.
Are there tools to help pharmacists avoid mistakes?
Yes. Software like PharmScript and Compounding.io automatically checks calculations, flags incompatible ingredients, and generates electronic batch records. Barcode scanners confirm ingredient identity before mixing. AI tools like CompoundingGuard AI cut calculation errors by 87% in pilot studies. But these tools only work if staff use them every time - no shortcuts, no disabling alerts.


