Every time you grab a bottle of pain reliever, cold medicine, or antacid off the shelf, you’re making a decision based on what’s written on the label. But here’s the truth: active ingredients are the only part that actually does the work. The rest-brand names, colors, shapes, and fancy packaging-is just noise. If you don’t know what’s inside, you could be risking your health without even realizing it.
What Exactly Is an Active Ingredient?
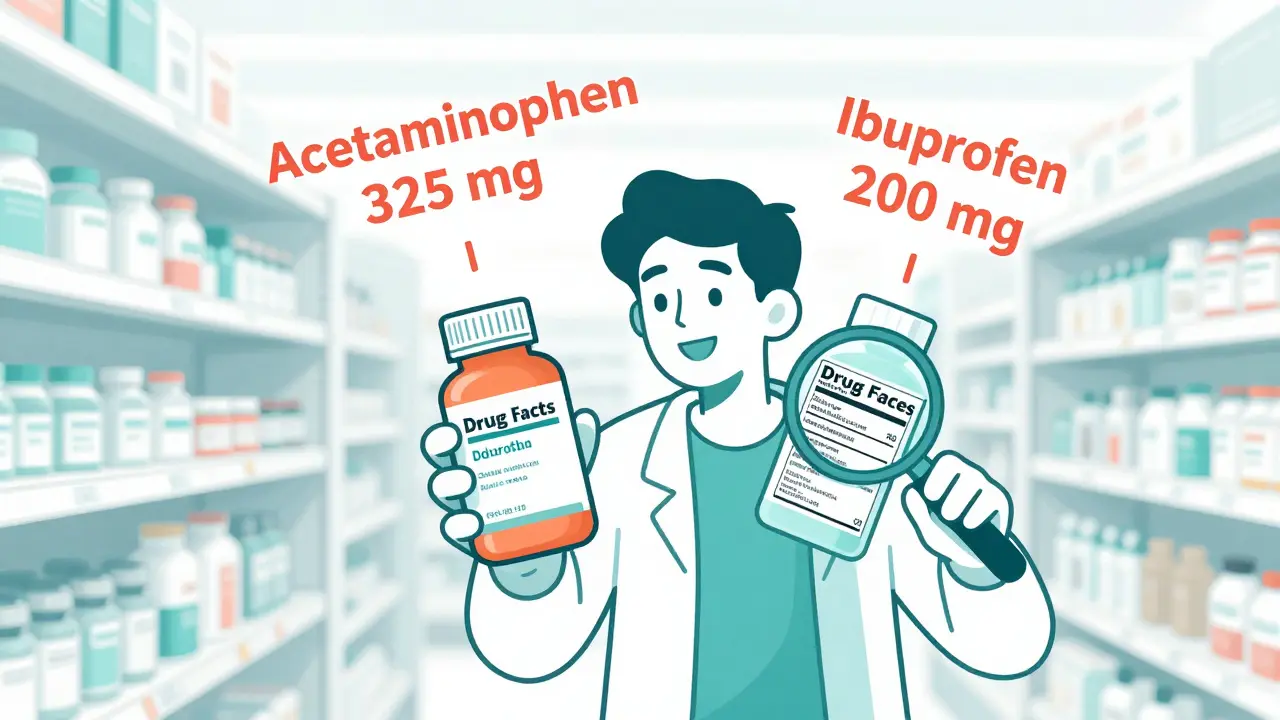
An active ingredient is the chemical in a medicine that causes the effect you want. It’s not the flavor, the dye, or the filler. It’s the part that reduces fever, relieves pain, stops a runny nose, or calms an upset stomach. Every OTC drug must list its active ingredients first on the Drug Facts label, along with the exact amount per dose. For example: acetaminophen 325 mg or ibuprofen 200 mg. That number matters. Too little, and it won’t work. Too much, and you could damage your liver or kidneys.The U.S. Food and Drug Administration (FDA) has strict rules about what active ingredients can be sold over the counter. Since 1972, they’ve reviewed more than 800 ingredients and grouped them into 107 categories called “monographs.” These are like rulebooks that say: “This ingredient is safe and effective for this use, at this dose.” If a product doesn’t follow the monograph, it’s not allowed on the shelf.
The Drug Facts Label: Your Best Friend
The Drug Facts label isn’t just a formality-it’s your safety guide. It’s required on every OTC medicine sold in the U.S. since 1999. There are seven sections, but only one matters most: Active Ingredient. Here’s how to read it:- Look for the word “Active Ingredient(s)” at the top of the list.
- Each ingredient is listed with its generic name and exact amount per dose (e.g., diphenhydramine HCl 25 mg).
- Ingredients are listed in order of amount-from most to least.
- Never see percentages like “5%” on internal meds-that’s only allowed for topical creams.
Many people miss this: if you’re taking two or more OTC medicines, you might be doubling your dose. For example, Tylenol, TheraFlu, and NyQuil all contain acetaminophen. If you take one of each, you could hit 1,950 mg in a single night-close to the daily limit of 4,000 mg. That’s how liver damage starts.
Top 5 Active Ingredients You Should Know
You don’t need to memorize all 800+ OTC ingredients. But these five show up in nearly 75% of all products:- Acetaminophen - Pain and fever reducer. Found in Tylenol, Excedrin, NyQuil, and many cold meds. Max daily dose: 4,000 mg for adults. Never exceed this. It’s the #1 cause of accidental liver failure in the U.S.
- Ibuprofen - Anti-inflammatory pain reliever. Found in Advil, Motrin, and store brands. Max daily dose: 1,200 mg for OTC. Can irritate your stomach if taken on an empty stomach or for too long.
- Diphenhydramine - Antihistamine that causes drowsiness. Used in Benadryl, Unisom, and nighttime cold formulas. Can cause confusion in older adults and dry mouth in everyone.
- Pseudoephedrine - Nasal decongestant. Found in Sudafed. Can raise blood pressure and cause jitteriness. Sold behind the pharmacy counter in many states.
- Dextromethorphan - Cough suppressant. Found in Robitussin, Delsym, and many cough syrups. Misused recreationally at high doses-some teens don’t realize it’s in their medicine cabinet.
Here’s the catch: different brands use the same active ingredient. Tylenol and Equate both have acetaminophen. Advil and CVS Health both have ibuprofen. The only difference? Price and packaging.
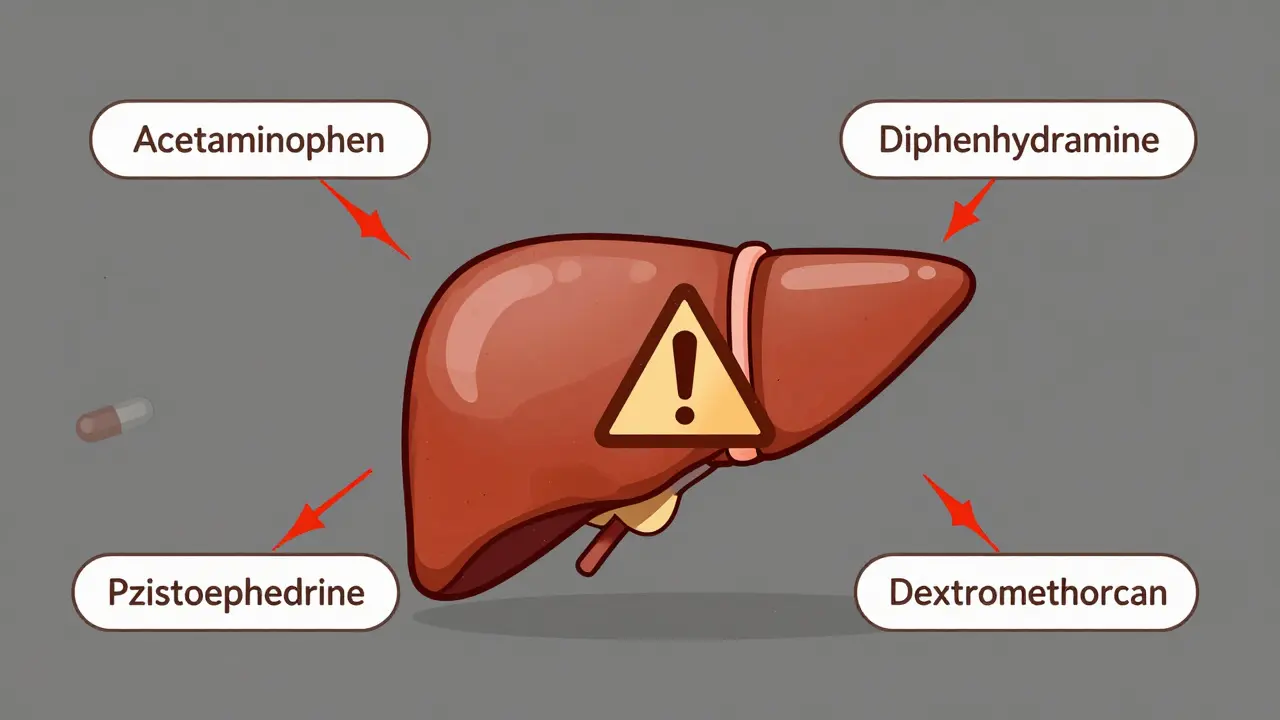
Why Brand Names Are Traps
You’ve seen the ads: “New! Triple Action Cold Relief!” Sounds unique, right? But if you check the label, it’s probably just acetaminophen, dextromethorphan, and phenylephrine-the same as the $5 store brand. Companies spend millions on branding so you think you’re buying something better. You’re not.A 2023 Consumer Reports survey found that 63% of adults couldn’t identify the active ingredient just by the product name. Only 28% knew that Aleve contains naproxen sodium. Meanwhile, 72% correctly guessed Tylenol = acetaminophen. Why? Because Tylenol is the most advertised. The rest? You have to read the label.
And here’s the scary part: if you’re on blood pressure meds, taking a cold medicine with phenylephrine could spike your pressure dangerously. If you have liver disease, acetaminophen could be deadly. If you’re pregnant, some antihistamines aren’t safe. None of that is on the front of the box. Only the Drug Facts label tells you.
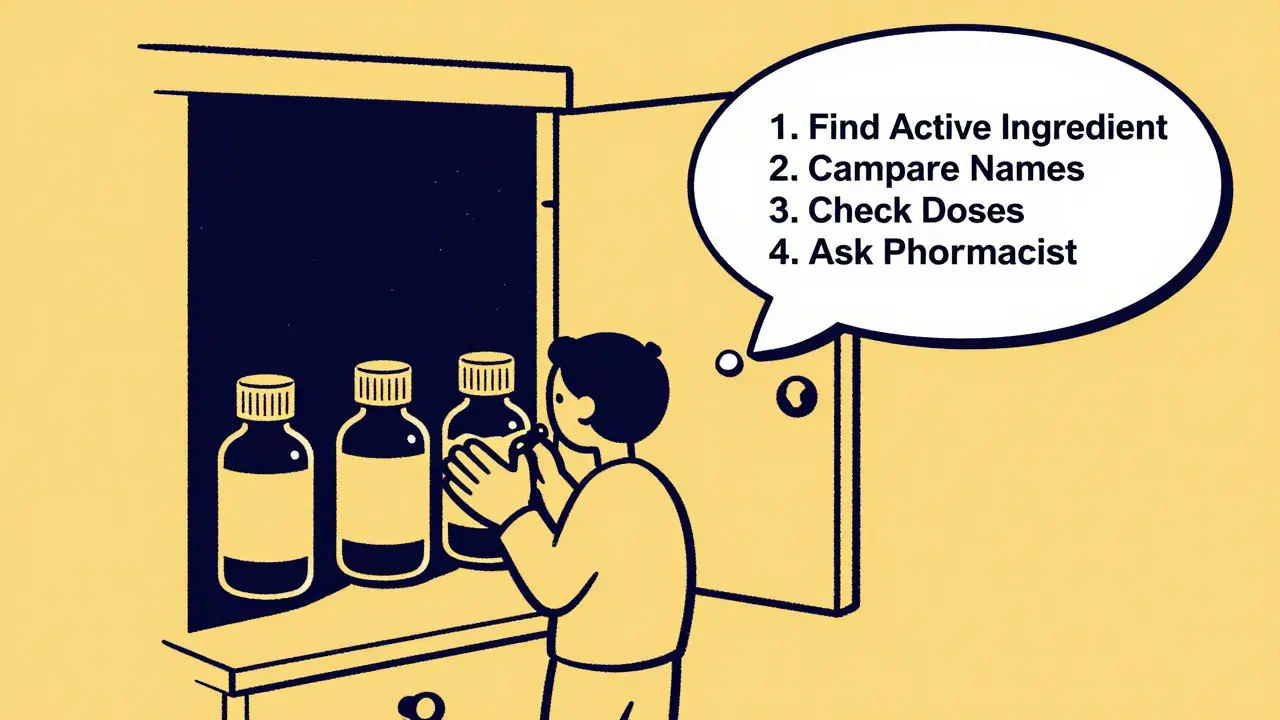
How to Avoid Overdose and Interactions
Accidental overdose is the #1 reason people end up in the ER because of OTC meds. Most of the time, it’s because they didn’t check the active ingredients. Here’s how to protect yourself:- Write it down. When you buy multiple OTC products, write each active ingredient on a piece of paper. If acetaminophen appears twice, you’re doubling up.
- Use the same brand for the same symptom. If you take ibuprofen for headaches, don’t switch to naproxen unless you know how they differ.
- Check your prescriptions. Some prescription painkillers also contain acetaminophen. Taking them with Tylenol can be dangerous.
- Ask your pharmacist. They’re trained to catch these overlaps. No judgment. Just ask: “Does this interact with my other meds?”
One Reddit user shared how they took TheraFlu Nighttime and two Tylenol tablets-thinking they were safe-then ended up in the ER with liver toxicity. They didn’t know both had acetaminophen. That story isn’t rare.
What’s Changing in 2026
The FDA is pushing for digital labels. By 2026, most OTC products will have QR codes that link to full ingredient details-including inactive ingredients like dyes and preservatives. This is huge for people with allergies. Right now, many don’t realize red dye #40 or cornstarch can trigger reactions.The CARES Act of 2020 forced the FDA to finalize all OTC monographs by December 2023. That means stricter rules on what can be sold. For example, loperamide (Imodium) is now under tighter control because of opioid abuse. Some products may be pulled from shelves if they don’t meet the new standards.
Meanwhile, the Consumer Healthcare Products Association launched “Know Your Active Ingredients” in January 2023. Retailers in 12 major chains now display posters showing common active ingredients and their uses. Since then, acetaminophen-related ER visits dropped 19% in those stores.
What You Should Do Today
You don’t need to be a pharmacist to use OTC drugs safely. Just do this:- Before buying, pick up the bottle and flip it over.
- Find the “Active Ingredient” section.
- Read the name and number out loud.
- Ask yourself: “Do I already have this in another medicine?”
- If unsure, walk to the pharmacy counter and ask.
It takes 45 seconds. That’s less time than scrolling through social media. But it could save your life-or your child’s.
Next time you reach for an OTC medicine, remember: the label isn’t there to sell you something. It’s there to protect you. Ignore it, and you’re gambling with your health. Read it, and you’re in control.
What’s the difference between active and inactive ingredients?
Active ingredients are the chemicals that treat your symptoms-like acetaminophen for pain or diphenhydramine for allergies. Inactive ingredients are fillers, dyes, flavors, or preservatives that help make the medicine stable or easier to swallow. They don’t treat anything, but they can cause allergic reactions. If you’re sensitive to red dye, corn, or gluten, check the “Inactive Ingredients” section on the label.
Can I take two OTC medicines at once?
Only if you’ve checked the active ingredients. Many cold, flu, and pain meds contain the same active ingredient. Taking two with acetaminophen, for example, can lead to overdose. Always write down each active ingredient and compare them. If they match, don’t take both.
Why do some OTC medicines have lower doses than prescription ones?
OTC medicines are designed for short-term, self-managed use. Prescription versions are stronger because they’re meant for longer use under a doctor’s supervision. For example, OTC ibuprofen is capped at 200 mg per tablet, while prescriptions go up to 800 mg. This keeps the risk low for people who don’t have medical oversight.
Is generic OTC medicine just as good as brand name?
Yes-by law, they must contain the same active ingredient, in the same amount, and work the same way. The only differences are the inactive ingredients (like flavor or dye), packaging, and price. Generic versions are often 50-80% cheaper. Always check the Drug Facts label to confirm the active ingredient matches.
What should I do if I think I’ve taken too much?
If you suspect an overdose-especially with acetaminophen, ibuprofen, or dextromethorphan-call Poison Control at 1-800-222-1222 immediately. Don’t wait for symptoms. Liver damage from acetaminophen can start without warning. Keep this number saved in your phone. It’s free, confidential, and available 24/7.
Next Steps
Start today: go to your medicine cabinet. Pull out three OTC products. Read the “Active Ingredient” section on each. Write them down. Do any repeat? If yes, you’ve found a potential risk. Next time you shop, bring your list. Compare it to the new product. You’ll save money, avoid side effects, and stay safe.Medicines aren’t candy. They’re powerful chemicals. Treat them like tools-read the instructions, use the right one, and never guess.


Jay Tejada
January 3, 2026 at 22:29Been there. Took NyQuil and Advil because ‘why not?’ Turned out I hit 3,000 mg of acetaminophen before bed. Woke up feeling like my liver was judging me. Now I check labels like I’m decoding a spy message. Worth it.
Allen Ye
January 5, 2026 at 18:51What’s fascinating isn’t just the chemistry-it’s the sociology of consumer manipulation. The FDA monographs are essentially a democratic compromise: public safety vs. corporate profit. We’ve outsourced our medical literacy to branding, and now we’re surprised when the system fails us. The real tragedy? People don’t realize they’re not buying medicine-they’re buying a narrative. Tylenol isn’t better than Equate. It’s just better at advertising. We’ve turned pharmacology into a reality TV show where the product is the villain and the label is the truth-teller we refuse to listen to.
Justin Lowans
January 6, 2026 at 10:16Brilliant breakdown. The Drug Facts label is one of the most underappreciated public health tools we have. I’ve started carrying a small notepad in my purse just to jot down active ingredients when I’m shopping. It’s transformed how I approach OTC meds-no more impulse buys, no more guessing. Knowledge isn’t just power; it’s prevention.
Michael Rudge
January 6, 2026 at 15:18Of course you didn’t know diphenhydramine causes confusion in the elderly-because you probably don’t know how to read. Why does the average adult still treat medicine like a lottery ticket? I’ve seen grandparents take three different cold pills because ‘they all say they help.’ You don’t need a PhD to understand ‘Acetaminophen: 500 mg.’ You need to stop being lazy. And yes, I’m talking to you.
Jack Wernet
January 7, 2026 at 16:24The structural integrity of the OTC regulatory framework is often overlooked. The monograph system, while imperfect, represents a rare instance of evidence-based policy implementation at scale. It is imperative that consumers recognize the FDA’s role not as a gatekeeper, but as a custodian of public health standards in an unregulated marketplace.
bob bob
January 7, 2026 at 18:49Y’all need to chill and just read the label. Seriously. It’s not rocket science. I showed my mom how to check ingredients last week-she was so proud she took a selfie with her medicine cabinet. We’re all just one 45-second check away from being safer.
Abhishek Mondal
January 7, 2026 at 21:56....And yet, you still don’t mention that pseudoephedrine is restricted because it’s used to make meth-so the government, in its infinite wisdom, punishes the innocent, not the criminals... and you call this ‘safety’? ...Also, why is dextromethorphan still legal at all? ...It’s not a cough suppressant-it’s a dissociative... ...and you’re telling me it’s safe for teens? ...Really? ...
Oluwapelumi Yakubu
January 9, 2026 at 06:03Bro, in Lagos, we just ask the pharmacist: ‘Which one for fever?’ They give us the cheapest one with paracetamol. No labels, no QR codes, no confusion. You folks overthink everything. Medicine is medicine. If it works, it works. Stop reading, start living. Also, my cousin took ten paracetamol once-he’s fine. You’re all too scared to live.
en Max
January 10, 2026 at 17:03It is imperative to recognize the ontological distinction between active and inactive constituents within pharmaceutical formulations. The regulatory compliance of the Drug Facts Label, as codified under 21 CFR § 201.66, represents a non-negotiable epistemic standard for consumer autonomy. Failure to engage with this framework constitutes a systemic epistemic negligence.
saurabh singh
January 12, 2026 at 09:52My uncle in Delhi used to say: ‘If it’s in a bottle, it’s not magic-it’s math.’ I never thought about it till I moved to the States and saw people paying $12 for ‘Advanced Cold Relief’ that had the same stuff as the $2 generic. Now I only buy store brands. Saved me $300 a year. And yeah, I read the label. Always. Simple. Smart. Safe.
Dee Humprey
January 13, 2026 at 07:50Just saved my husband’s life last month. He was taking Advil and a store-brand cold med. Both had ibuprofen. He got stomach bleeding. I didn’t know until I checked the label. Now I have a sticky note on our medicine cabinet: ‘CHECK THE ACTIVE INGREDIENTS.’ I’m not even a nurse. Just someone who cares. 🙏
John Wilmerding
January 14, 2026 at 16:27The 2026 QR code initiative is a necessary evolution in pharmaceutical transparency. While the current monograph system provides a foundational framework, the integration of digital metadata-particularly regarding allergens and excipients-will significantly reduce iatrogenic harm. This represents a paradigm shift from reactive regulation to proactive consumer empowerment.