Key Takeaways
- Genetic factors account for roughly 10‑15% of Parkinson's cases, with several high‑impact genes identified.
- LRRK2, SNCA, PARK2, PINK1 and DJ-1 are the most frequently implicated genes.
- Genetic testing is recommended for early‑onset patients, those with a strong family history, or when trial eligibility requires a known mutation.
- Understanding a patient’s genetic profile can guide treatment choices, inform family members, and open doors to targeted clinical trials.
- Research is moving toward gene‑editing and biomarker‑driven therapies, but lifestyle and environmental factors remain crucial.
What Is Parkinson's Disease?
Parkinson's disease is a progressive neurodegenerative disorder characterized by the loss of dopamine‑producing neurons in the substantia nigra, leading to motor symptoms such as tremor, rigidity, bradykinesia, and postural instability. Non‑motor signs-sleep disturbances, constipation, mood changes-often appear years before movement problems, making early detection challenging.
The disease affects about 1 % of people over 60, and its prevalence rises sharply with age. While most cases are sporadic, a growing body of evidence shows that genetics play a critical role in a minority of patients, shaping both susceptibility and disease trajectory.
How Genetics Influences Parkinson's Disease
When researchers talk about "genetic Parkinson's," they refer to instances where inherited DNA variations meaningfully increase risk. These variations can be single‑point mutations, small insertions/deletions, or larger copy‑number changes. Unlike classic Mendelian diseases, Parkinson's genetics is a mosaic of high‑penetrance mutations (rare but powerful) and low‑penetrance risk alleles that modestly shift the odds.
Genome‑wide association studies (GWAS) have identified more than 90 loci linked to Parkinson's risk. However, five genes dominate clinical awareness because their mutations often lead to a clear, hereditary pattern. Understanding these genes helps clinicians decide who should be offered testing and what counseling is needed.
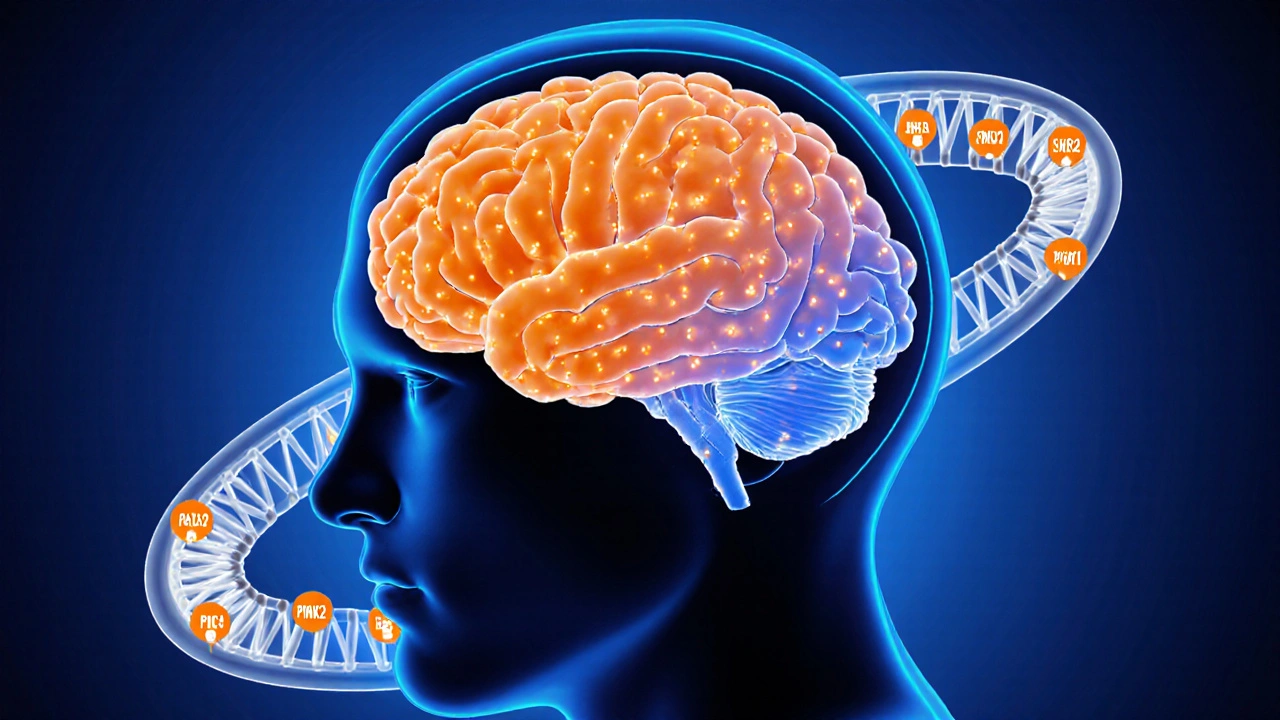
Major Genes Linked to Parkinson's Disease
The following table summarizes the most relevant genes, their inheritance mode, typical age of onset, and hallmark clinical features. Only the first mention of each gene includes microdata markup.
| Gene | Inheritance | Typical Onset (years) | Core Clinical Clues |
|---|---|---|---|
| LRRK2 (leucine‑rich repeat kinase 2) | Autosomal dominant | 55‑70 | Similar to idiopathic PD, often good response to levodopa |
| SNCA (alpha‑synuclein) | Autosomal dominant | 30‑50 | Rapid progression, early dysautonomia, dementia |
| PARK2 (Parkin) | Autosomal recessive | 15‑30 | Predominantly motor signs, slower disease course |
| PINK1 | Autosomal recessive | 20‑40 | Early onset, good levodopa response, limited non‑motor symptoms |
| DJ-1 (PARK7) | Autosomal recessive | 25‑45 | Motor symptoms plus oxidative stress markers in labs |
These genes account for the majority of familial cases. For instance, LRRK2 mutations appear in up to 5 % of Caucasian patients with a family history, while PARK2 is the leading cause of early‑onset Parkinson's under age 30.
Genetic Testing: Who Should Consider It?
Genetic testing is no longer a niche service; many neurology clinics in Australia and abroad now offer panel tests covering the five major genes plus additional risk loci. Testing is most useful in three scenarios:
- Early‑onset disease (≤50 years). A younger diagnosis often signals a hereditary form.
- Positive family history. Two or more first‑degree relatives with Parkinson's or related movement disorders raise the pre‑test probability.
- Clinical trial eligibility. Some novel therapies target LRRK2 or alpha‑synuclein; trial sponsors require confirmed mutations.
When ordering a test, clinicians should involve a certified genetic counselor with expertise in neurodegenerative diseases. The counselor explains potential outcomes, privacy considerations, and the impact on insurance.
Interpreting Risk and Family Planning
Finding a pathogenic mutation changes the risk calculus for relatives. For autosomal dominant genes like LRRK2 and SNCA, each child has a 50 % chance of inheriting the variant. However, penetrance varies: only about 30‑40 % of LRRK2 carriers develop symptoms by age 80, meaning many never become patients.
Recessive genes (PARK2, PINK1, DJ-1) require two defective copies. Carriers (heterozygotes) usually remain symptom‑free, but when both parents are carriers there’s a 25 % chance each child will be affected.
Reproductive options include:
- Pre‑implantation genetic testing (PGT‑M) for couples undergoing IVF.
- Prenatal testing via chorionic villus sampling or amniocentesis, though ethical considerations apply.
- Adoption or donor gametes if the family wishes to avoid transmission entirely.
Importantly, a positive test does not guarantee disease, and lifestyle modifications (exercise, caffeine intake, avoidance of pesticides) may still lower overall risk.

Current Research and Future Directions
Scientists are leveraging CRISPR‑Cas9 to edit pathogenic LRRK2 and SNCA mutations in cell and animal models. Early results show reduced alpha‑synuclein aggregation and improved motor function, but human trials are years away.
Another promising avenue is the development of biomarkers that reflect genetic risk. For example, blood‑based assays measuring phosphorylated alpha‑synuclein could identify carriers before clinical signs appear, enabling early intervention.
Large international consortia-such as the Global Parkinson's Genetics Program-are pooling GWAS data to pinpoint additional low‑penetrance loci. These efforts aim to create polygenic risk scores that combine dozens of variants into a single risk number, similar to what cardiology does for heart disease.
Practical Steps for Patients and Clinicians
Whether you’re a newly diagnosed patient, a family member, or a neurologist, here’s a quick checklist to turn genetic knowledge into action:
- Take a detailed family history. Note ages at diagnosis, symptoms, and any related conditions (e.g., dementia, REM sleep behavior disorder).
- Discuss testing with a specialist. If you’re under 50 or have multiple affected relatives, ask your neurologist about a multi‑gene panel.
- Engage a genetic counselor. They’ll walk you through possible results, insurance implications, and options for relatives.
- Integrate results into care. A confirmed LRRK2 mutation may qualify you for a kinase‑inhibitor trial; a PARK2 result may suggest a more favorable prognosis and influence medication choice.
- Adopt neuroprotective lifestyle habits. Regular aerobic exercise, a Mediterranean‑style diet, and avoidance of neurotoxins (pesticides, heavy metals) are supported by epidemiologic data.
- Stay updated. The genetics field evolves rapidly; consider annual check‑ins with your care team to re‑evaluate eligibility for emerging therapies.
Remember, genetics is just one piece of the puzzle. Combining genetic insight with clinical assessment, lifestyle, and emerging treatments offers the best chance for a personalized approach.
Frequently Asked Questions
Can anyone with Parkinson's disease get a genetic test?
Testing is most informative for people diagnosed before age 50, those with a clear family history, or when a clinical trial requires a known mutation. For typical late‑onset cases without a family history, the yield is low, but patients can still opt for testing after counseling.
If I carry an LRRK2 mutation, will I definitely develop Parkinson's?
No. Penetrance for LRRK2 varies between 30 % and 70 % depending on the specific variant and environmental factors. Many carriers remain symptom‑free throughout life.
Are there treatments that target the genetic cause?
A few early‑phase trials are testing LRRK2 kinase inhibitors and antisense oligonucleotides aimed at reducing alpha‑synuclein production. While results are promising, these therapies are not yet commercially available.
How does genetic testing affect insurance in Australia?
Under the Genetic Discrimination Act (2003), insurers cannot use genetic test results to refuse coverage or increase premiums for health insurance. However, life insurance policies are not covered by this legislation, so it’s wise to discuss potential implications with a counselor.
What lifestyle changes can lower my genetic risk?
Regular aerobic exercise (150 minutes per week), a diet rich in fruits, vegetables, and omega‑3 fatty acids, adequate sleep, and avoidance of environmental toxins (pesticides, heavy metals) have all been linked to reduced Parkinson’s incidence, even among genetically predisposed individuals.

Holly Green
October 22, 2025 at 16:07Genetics gives us a useful roadmap, but lifestyle still matters. Testing should be reserved for cases where it truly changes management.
Oliver Johnson
October 22, 2025 at 18:54What the US healthcare system fails to see is that genetic testing is a waste of money for most patients. We should focus on proven therapies instead.
Taylor Haven
October 22, 2025 at 21:41The narrative that genetics is a panacea for Parkinson's disease is a carefully crafted illusion. From the moment the first GWAS results were published, a hidden agenda has been pushing a biotech agenda that benefits a select few. These corporations fund the research, own the patents, and then sell expensive panels to desperate patients. What they conveniently forget to mention is that the majority of carriers never develop symptoms, yet they are billed for unnecessary follow‑up. The regulatory bodies are in on it, turning a profit off the fear of hereditary doom. Meanwhile, the real environmental culprits-pesticides, heavy metals, dietary toxins-are brushed aside as ‘minor factors’ in the press releases. It is no coincidence that the same labs that market the tests also lobby for stricter pesticide regulations that would hurt their sponsors. Patients are told to ‘stay vigilant’ while the system quietly silences any research that points to non‑genetic interventions. I have spoken with several clinicians who admit they feel pressured to order panels in order to stay competitive in the research grant race. The moral compromise is evident, and it betrays the trust of families who hope a simple genetic answer will give them control. Even the notion of pre‑implantation genetic testing is weaponized to create a market for IVF clinics that collaborate with test manufacturers. Do we really need to edit embryos when we can teach patients to avoid known neurotoxins? The promise of CRISPR cures is an alluring science‑fiction story that distracts from the immediate, low‑cost preventative measures. In short, the genetic hype is a distraction, a smokescreen that keeps power and profit out of reach of the average patient. I urge anyone reading this to question the sources, seek independent counsel, and consider lifestyle changes that have real evidence behind them. The future may indeed hold gene‑editing breakthroughs, but until then, the focus should be on transparency and accountability.
Gary Marks
October 23, 2025 at 00:27Alright, let’s cut through the nonsense. You think a single mutation decides a person’s fate? That’s a cartoon plot, not science. The reality is a tangled web of genes, environment, and sheer bad luck, and you’re trying to sell a one‑size‑fits‑all test like it’s a miracle cure. I’ve seen patients shell out thousands for a piece of paper while ignoring simple habits like exercise and diet that actually move the needle. If you want results, start by killing the toxins in your kitchen, not by chasing every new gene panel that flashes on a lab’s website. In the end, it’s the noisy hype that drowns out the quiet, proven steps we all should take.
Vandermolen Willis
October 23, 2025 at 03:14Great overview! I’ve learned that genetics can guide treatment, but staying active and eating well still matters. 😊 It’s good to see the checklist for patients and clinicians. 👍
Mary Keenan
October 23, 2025 at 06:01Genetic panels are overpriced junk.
Steven Young
October 23, 2025 at 08:47Look at how the labs and insurers collude to push expensive tests they control. The public is told it’s about precision medicine while the real goal is profit.
Kelly Brammer
October 23, 2025 at 11:34It is ethically irresponsible to market genetic testing without fully disclosing its limited predictive value.
Ben Collins
October 23, 2025 at 14:21Oh sure, because the next step after a blood test is definitely to hire a life‑coach for your mitochondria. But hey, if you enjoy paying for hype, go ahead.
Denver Bright
October 23, 2025 at 17:07I’ve been watching the discussion and felt compelled to share my own experience even though it’s not my story I think it adds perspective.
Kelli Benedik
October 23, 2025 at 19:54Wow, this thread is like a roller‑coaster of secrets and betrayals! 🎭🧬 I can’t help but feel the sting of every hidden agenda they’re trying to hide behind the science. It’s heartbreaking to see families torn apart by promises that sparkle like fireworks but fade into silence. 🌟💔 Yet there’s also a fierce hope that one day the truth will shine brighter than the glitter of corporate profit.